Scientists redid an experiment that showed how life on Earth could have started. They found a new possibility
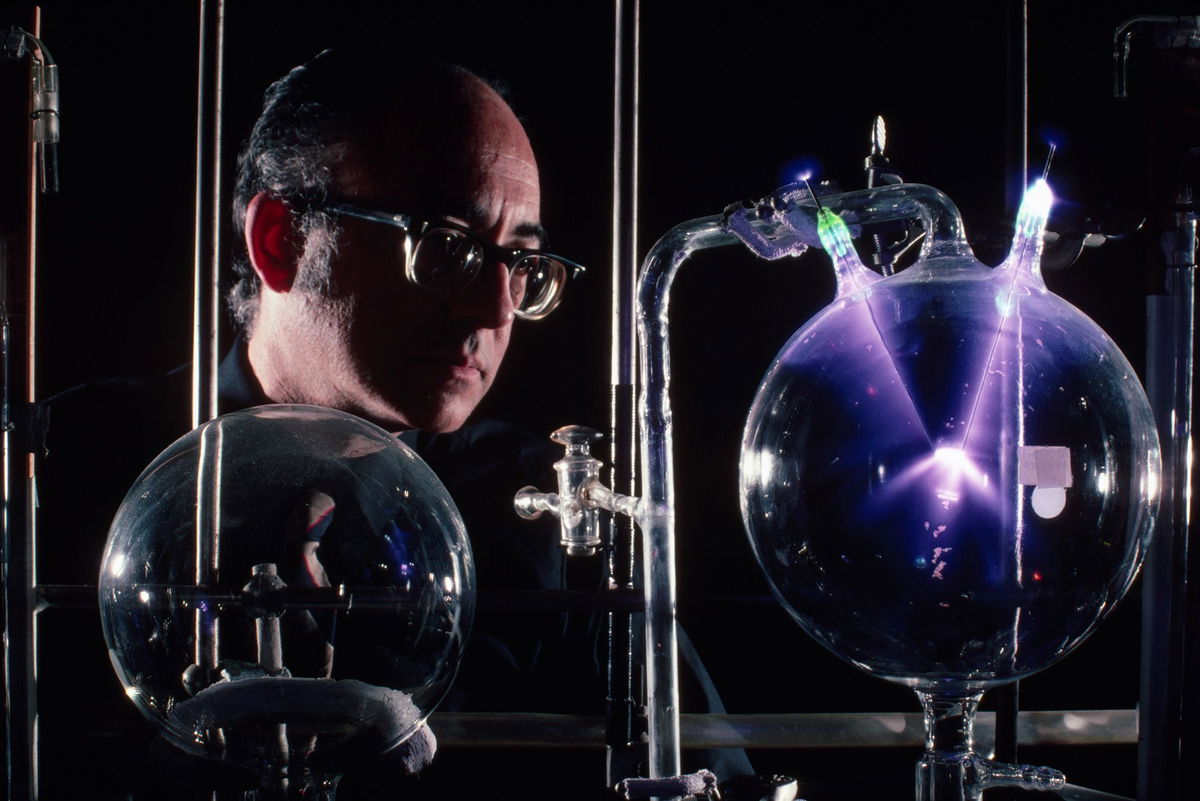
American chemist Stanley Miller
(CNN) — “It’s alive! IT’S ALIVE!”
In the 1931 movie “Frankenstein,” Dr. Henry Frankenstein howling his triumph was an electrifying moment in more ways than one. As massive bolts of lightning and energy crackled, Frankenstein’s monster stirred on a laboratory table, its corpse brought to life by the power of electricity.
Electrical energy may also have sparked the beginnings of life on Earth billions of years ago, though with a bit less scenery-chewing than that classic film scene.
Earth is around 4.5 billion years old, and the oldest direct fossil evidence of ancient life — stromatolites, or microscopic organisms preserved in layers known as microbial mats — is about 3.5 billion years old. However, some scientists suspect life originated even earlier, emerging from accumulated organic molecules in primitive bodies of water, a mixture sometimes referred to as primordial soup.
But where did that organic material come from in the first place? Researchers decades ago proposed that lightning caused chemical reactions in ancient Earth’s oceans and spontaneously produced the organic molecules.
Now, new research published March 14 in the journal Science Advances suggests that fizzes of barely visible “microlightning,” generated between charged droplets of water mist, could have been potent enough to cook up amino acids from inorganic material. Amino acids — organic molecules that combine to form proteins — are life’s most basic building blocks and would have been the first step toward the evolution of life.
“It’s recognized that an energetic catalyst was almost certainly required to facilitate some of the reactions on early Earth that led to the origin of life,” said astrobiologist and geobiologist Dr. Amy J. Williams, an associate professor in the department of geosciences at the University of Florida. For animo acids to form, they need nitrogen atoms that can bond with carbon. Freeing up atoms from nitrogen gas requires severing powerful molecular bonds and takes an enormous amount of energy, according to Williams, who was not involved in the research.
“Lightning, or in this case, microlightning, has the energy to break molecular bonds and therefore facilitate the generation of new molecules that are critical to the origin of life on Earth,” Williams told CNN in an email.
Mist and microlightning
To recreate a scenario that may have produced Earth’s first organic molecules, researchers built upon experiments from 1953 when American chemists Stanley Miller and Harold Urey concocted a gas mixture mimicking the atmosphere of ancient Earth. Miller and Urey combined ammonia (NH3), methane (CH4), hydrogen (H2) and water, enclosed their “atmosphere” inside a glass sphere and jolted it with electricity, producing simple amino acids containing carbon and nitrogen. The Miller-Urey experiment, as it is now known, supported the scientific theory of abiogenesis: that life could emerge from nonliving molecules.
For the new study, scientists revisited the 1953 experiments but directed their attention toward electrical activity on a smaller scale, said senior study author Dr. Richard Zare, the Marguerite Blake Wilbur Professor of Natural Science and professor of chemistry at Stanford University in California. Zare and his colleagues looked at electricity exchange between charged water droplets measuring between 1 micron and 20 microns in diameter. (The width of a human hair is 100 microns.)
“The big droplets are positively charged. The little droplets are negatively charged,” Zare told CNN. “When droplets that have opposite charges are close together, electrons can jump from the negatively charged droplet to the positively charged droplet.”
The researchers mixed ammonia, carbon dioxide, methane and nitrogen in a glass bulb, then sprayed the gases with water mist, using a high-speed camera to capture faint flashes of microlightning in the vapor. When they examined the bulb’s contents, they found organic molecules with carbon-nitrogen bonds. These included the amino acid glycine and uracil, a nucleotide base in RNA.
“We discovered no new chemistry; we have actually reproduced all the chemistry that Miller and Urey did in 1953,” Zare said. Nor did the team discover new physics, he added — the experiments were based on known principles of electrostatics.
“What we have done, for the first time, is we have seen that little droplets, when they’re formed from water, actually emit light and get this spark,” Zare said. “That’s new. And that spark causes all types of chemical transformations.”
Water and life
Lightning is a dramatic display of electrical power, but it is also sporadic and unpredictable. Even on a volatile Earth billions of years ago, lightning may have been too infrequent to produce amino acids in quantities sufficient for life — a fact that has cast doubt on such theories in the past, Zare said.
Water spray, however, would have been more common than lightning. A more likely scenario is that mist-generated microlightning constantly zapped amino acids into existence from pools and puddles, where the molecules could accumulate and form more complex molecules, eventually leading to the evolution of life.
“Microdischarges between obviously charged water microdroplets make all the organic molecules observed previously in the Miller-Urey experiment,” Zare said. “We propose that this is a new mechanism for the prebiotic synthesis of molecules that constitute the building blocks of life.”
However, even with the new findings about microlightning, questions remain about life’s origins, he added. While some scientists support the notion of electrically charged beginnings for life’s earliest building blocks, an alternative abiogenesis hypothesis proposes that Earth’s first amino acids were cooked up around hydrothermal vents on the seafloor, produced by a combination of seawater, hydrogen-rich fluids and extreme pressure.
Yet another hypothesis suggests that organic molecules didn’t originate on Earth at all. Rather, they formed in space and were carried here by comets or fragments of asteroids, a process known as panspermia.
“We still don’t know the answer to this question,” Zare said. “But I think we’re closer to understanding something more about what could have happened.”
Though the details of life’s origins on Earth may never be fully explained, “this study provides another avenue for the formation of molecules crucial to the origin of life,” Williams said. “Water is a ubiquitous aspect of our world, giving rise to the moniker ‘Blue Marble’ to describe the Earth from space. Perhaps the falling of water, the most crucial element that sustains us, also played a greater role in the origin of life on Earth than we previously recognized.”
Mindy Weisberger is a science writer and media producer whose work has appeared in Live Science, Scientific American and How It Works magazine.
The-CNN-Wire
™ & © 2025 Cable News Network, Inc., a Warner Bros. Discovery Company. All rights reserved.