OSU research about how bacteria communication could open door to new infection-fighting medicines
CORVALLIS, Ore. (KTVZ) – Oregon State University scientists have identified proteins that prevent a bacterial cell from becoming misguided by its own messaging, allowing it to instead wait for collective communication from its group.
The research is important because understanding this type of signaling, known as quorum sensing and integral to bacterial pathogens, opens the door to potential new drugs that can disrupt it and thwart infection.
Findings were published Monday in the Proceedings of the National Academy of Sciences.
Martin Schuster, a professor in OSU's Department of Microbiology in the colleges of Science and Agricultural Sciences, and doctoral student Parker Smith study quorum sensing in the pathogen Pseudomonas aeruginosa, a gram-negative bacterium that displays a variety of social behaviors.
P. aeruginosa, a common cause of lung and wound infections among hospital patients and people with weakened immune systems, is a model organism for quorum sensing research with a well understood signaling circuit, the scientists said.
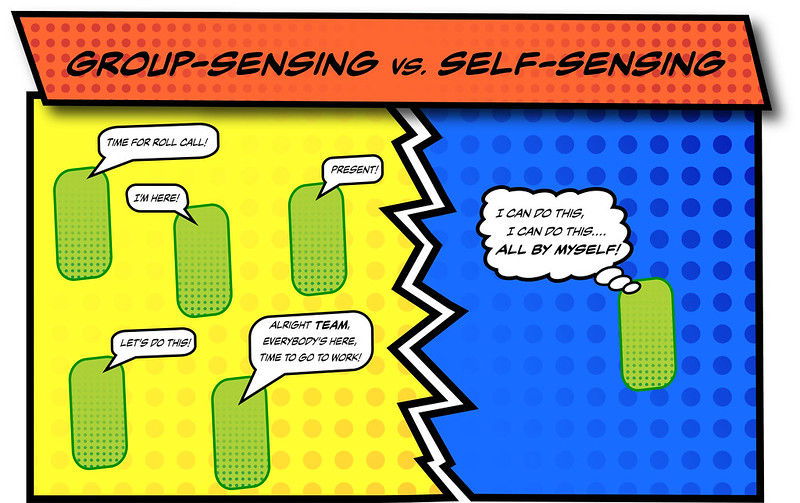
“Sometimes single-celled organisms need to work together with other cells,” Schuster said. “Bacteria and other single-celled microbes can coordinate behaviors and act as a group via quorum sensing, in which cells produce and sense a small chemical signal that is shared within the population.”
As the signal is released from cells and reaches a high enough concentration in their environment, a quorum is achieved – certain genes are simultaneously activated and specific group behaviors are set in motion, Smith said.
It’s a strength-in-numbers approach that allows bacteria to join forces to do things they could not do by themselves, like causing infection in animals and plants, acquiring certain nutrients and competing against other microbes.
“Bacterial infection often involves toxins that only harm the host at high levels, when produced by all bacterial cells at once,” Smith said.
A major unresolved question about quorum sensing, the researchers said, has been why the signal that’s produced inside an individual cell is not sensed by that same cell before it is released, spurring the cell into premature, solo action.
“In essence, what prevents signal ‘short-circuiting’ from happening?” Schuster said. “Our research addresses this question that’s fundamental to our understanding of quorum sensing.”
Smith and Schuster learned that a set of proteins called antiactivators are crucial for short-circuit prevention. The proteins work as a quorum sensing “tuner” by causing cells to be less sensitive to the quorum signal.
The researchers developed bacterial strains that lacked two different types of antiactivator proteins and then looked at quorum sensing behaviors in individual cells.
“We found that without antiactivators, a fraction of cells in a P. aeruginosa population engaged in ‘self-talk,’” Smith said. “In these cells, signal short-circuiting had activated quorum-sensing-dependent behaviors at all times, irrespective of cell density and without any communication with other cells. Our research shows how bacteria put the brakes on quorum sensing to achieve true communication in a group.”
In addition to helping the quest for new antibiotics that can inhibit quorum sensing in bacterial pathogens, the findings also provide background knowledge useful for the engineering of cells with new properties in a field called synthetic biology, Schuster said.
The study was funded by the National Science Foundation.



